(Note: This entry has been written by Dr. Mike Bernstein - Thank you, Mike!)
It’s a “given” that for NMR the chemical shift must be reported relative to standard. The most widely used is the 1H signal of tetramethylsilane (TMS) in chloroform, which has an assigned value of exactly zero. This is convention, and we all adhere to it. Correctly referencing 1H NMR spectra is seldom a difficulty, whether we use co-dissolved TMS (or a water-soluble equivalent), or the residual proton signal from the deuterated solvent. Things can get more complex, but this works for the vast majority of us. The chemical shift, d, is defined thus:
Venturing to the “dark side” of NMR – nuclei other than 1H – seldom stretches beyond 13C for most, and a residual solvent signal is very often present that can be used as a secondary chemical shift reference. But beautiful possibilities tempt many of us. Whether you are interested in biomolecular NMR and live and breathe 15N and possibly 31P, or 2H, or an orgametallic chemist with an interest in far more exotic nuclei, each heteronucleus has its charms and challenges. One thing unites NMR of all nuclei: adherence to a convention for chemical shifts. This can be easier said than done, given that some reference materials are difficult to handle, expensive, etc. The chemical shift reference compound for 19F NMR is the banned substance, Freon-11.
Absolute chemical shifts
We get help from a group working under the IUPAC [1] guise for their work in helping us calculate the chemical shift scale for all NMR-active nuclei [2]. That is, they provide us with a standard way to get the chemical shift precisely correct for any and all heteronuclear NMR spectra. That’s amazing - and hugely useful!
So how does it work? Well, it’s quite simple, really. At the heart of the calculation is the absolute frequency of the 1H signal of TMS for your NMR spectrometer hardware (console, probe, etc.) and sample (solvent, temperature, etc.). You need to be able to determine the exact frequency of this reference signal to seven decimal figures, at least. The following equation applies (sometimes expressed as a percentage) and uses a ratio to describe a constant, X (Greek capital Xi):
Making it easy with Mnova
We make heavy use of absolute referencing
(AR) in Mnova, with the following available:
- Correctly reference an X-nucleus spectrum when the referenced 1H spectrum is available
- Apply AR to heteronuclear axes in 2D experiments
- Allow users to customise the X values
- Indirect 1H spectrum referencing using nTMS for a specific hardware and solvent (locked)
Referencing heteronuclear spectra
Ensure that you have a document having (a)
a correctly referenced 1H NMR spectrum, and (b) one or more –nucleus spectra.
Select Analysis è Reference è Absolute reference… and choose the X-axis spectrum/spectra to reference.
The table of X values
Note that by tapping on the “X values…” button you will be presented with the table of X-nuclei. In the case of 15N, for example, you can choose which reference standard you want to use. By clicking on the blue “+” button you can enter your own, customised value.
Referencing 2D spectra
When there are 2D spectra in the document then the Absolute reference… selection will reflect this, and allow you to choose which spectrum is used for referencing purposes, and the traces to which this should be applied. Note that you can adjust the referencing of 1H and X-nuclei.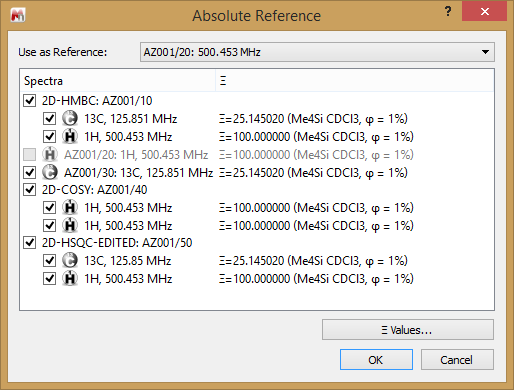
Referencing a 1H spectrum
You can use saved nTMS values to reference another 1H spectrum from the same NMR spectrometer. Start with a correctly-referenced 1H NMR spectrum, and select Analysis è Reference è Edit saved references… From this dialogue you can add the value for the particular hardware and measurement conditions – solvent, temperature, etc.
Now, when you select Analysis è Reference è Apply saved reference then the saved value will be used if the criteria are met.
Conclusions
Absolute referencing is a powerful way to ensure that data are correctly referenced. This is equally important in open-access environments as it is under automation, where it helps processes such as Verify be more robust.
References
[1] (a) Harris RK, Becker ED, Cabral de Menzes SM, Goodfellow R, Granger P. Pure Appl. Chem. 2001; 73: 1795
(b) Harris RK, Becker ED. J. Magn. Reson. 2003; 156: 323.
[2] This is nicely described here: http://www.chem.wisc.edu/~cic/nmr/Guides/Other/Xi_chem_shift_scale.pdf







No comments:
Post a Comment