Nothing new
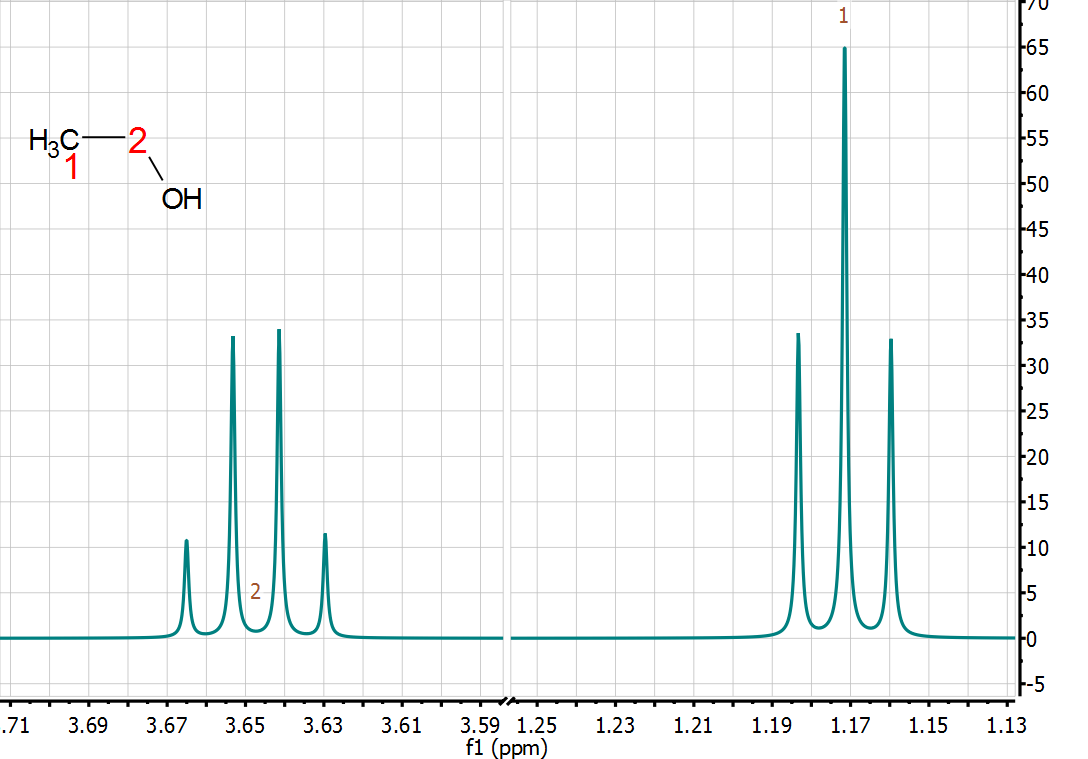
under the sun. This is a very simple spectrum where the two observed multiplets
seem to follow very nicely the well-known first order multiplet rules that most
chemists use on daily basis. In this case, a very simple A3X2 spin system.
But does
this mean that this spectrum is actually composed by only 7 peaks? The answer
is, of course not, there are many more peaks! But because of the very limited
resolution, most of them are not observed and merge in such a way that only 7
peaks are ultimately observed.
In other
words, the number of NMR transitions
is usually much larger than the number of peaks we actually observe in the spectrum.
Just to give an example: A molecule containing 30 coupled protons will result
in a spectrum having 16106127360 (=1.61E+10) transitions. As its corresponding NMR
spectrum will show only about 100-200 peaks, that makes it well over eighty
million quantum transitions per resolved peak!
For
example, let’s magnify the quadruplet and use Mnova unique capabilities to
display the individual transitions by simply hovering with the mouse cursor
over the atoms in the molecule (CH2 in this case). We can see that there are
some ‘hidden peaks’, these are the NMR transitions calculated by diagonalizing
the NMR Hamiltonian.
These transitions are so close that they cannot be resolved under the usual NMR resolution conditions. In fact, to separate all these signals, it would be necessary to have a spectral resolution of < 0.01 Hz
Whilst this
is far from being feasible experimentally nowadays, it is easy to do
numerically. In the figure below I’m displaying the same synthetic spectrum of
Ethanol but this time synthesized using a line width of just 0.01 Hz and 1 MB
of digital data points. Now the individual transitions can be seen as resolved
peaks so in this example a transition will be virtually equivalent to an NMR
peak.
Simply put,
an NMR spectrum is just a superposition of all spectral transitions (which can
be in the order of millions), transitions compose peaks, peaks group into
multiplets, and multiplets compose the spectrum.
The ability
of Mnova to show the individual NMR transitions in a synthetic spectrum can be
a good teaching tool
For a more theoretical and rigorous discussion on NMR transitions, see A.D. Bain, D.A. Fletcher and P. Hazendonk. "What is a transition?" Concepts in Magnetic Resonance 10 85- 98 (1998) (link)



No comments:
Post a Comment